![]()
![]()
AFMS partners with QIAGEN to provide solutions for syndromic testing - the QIAstat-Dx syndromic testing system,
A true Sample to Insight Solution
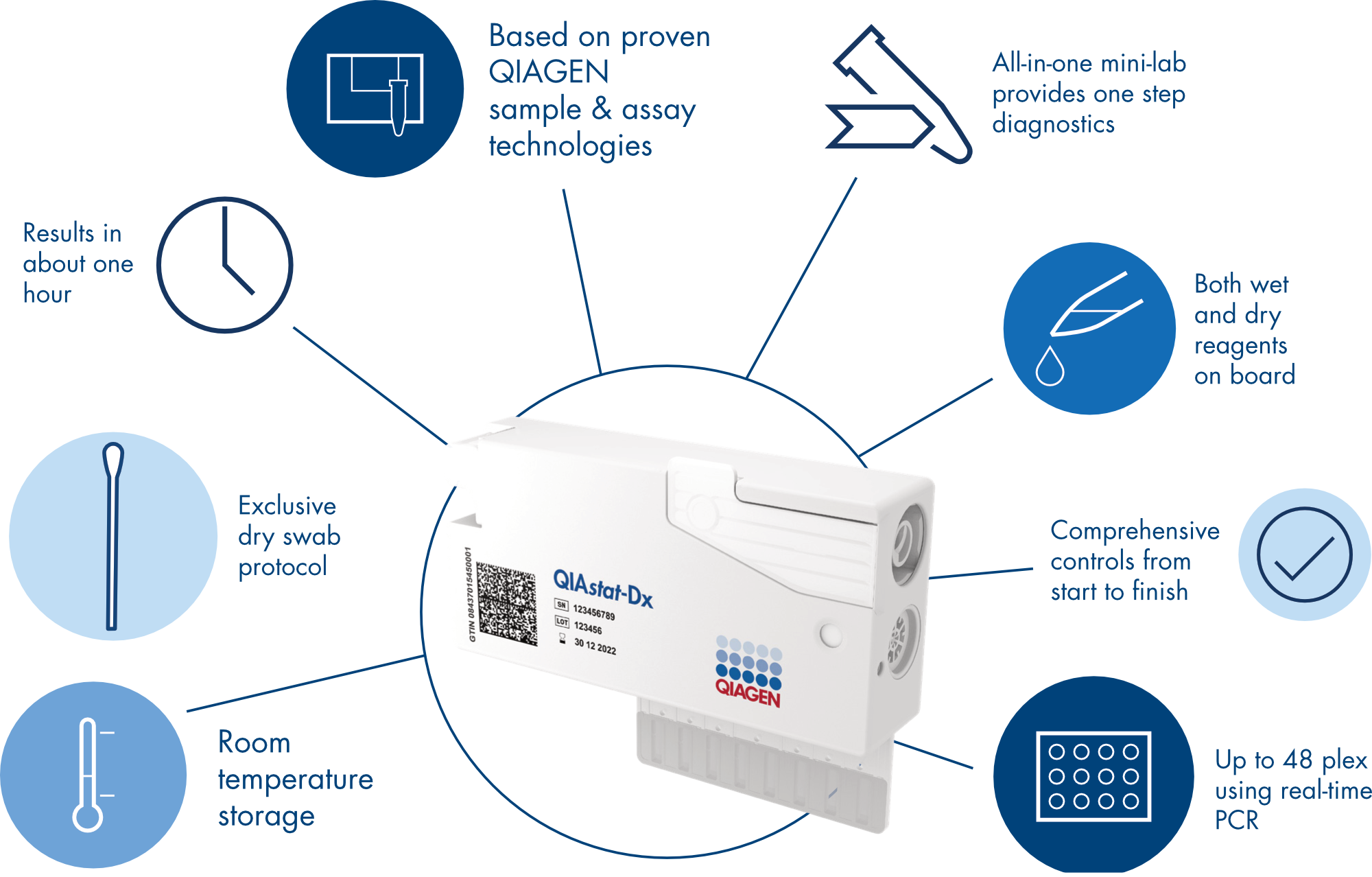
QIAstat-Dx simplifies infectious disease diagnostics so you can get faster results for your patients. All you need are a patient sample, a QIAstat-Dx assay cartridge and the intuitive QIAstat-Dx instrument.
The QIAstat-Dx assay cartridges use multiplex PCR technology – also known as syndromic testing – to quickly survey many different pathogens in a single patient sample. The cartridges come ready with all reagents preloaded and use QIAGEN's trusted assay chemistry to provide comprehensive results in about an hour.
The QIAstat-Dx Analyzer is a flexible modular system, perfect for labs of every size.
Each instrument is made up of 1 Operational Module, which contains the intuitive touch-screen interface, and between 1 through 4 Analytical Modules, letting you test up to four samples at once. The QIAstat-Dx Analyzer condenses dozens of pathogen real-time PCR tests into one small benchtop unit.
Simplify syndromic testing with QIAstat-Dx QIAstat-Dx Brochure
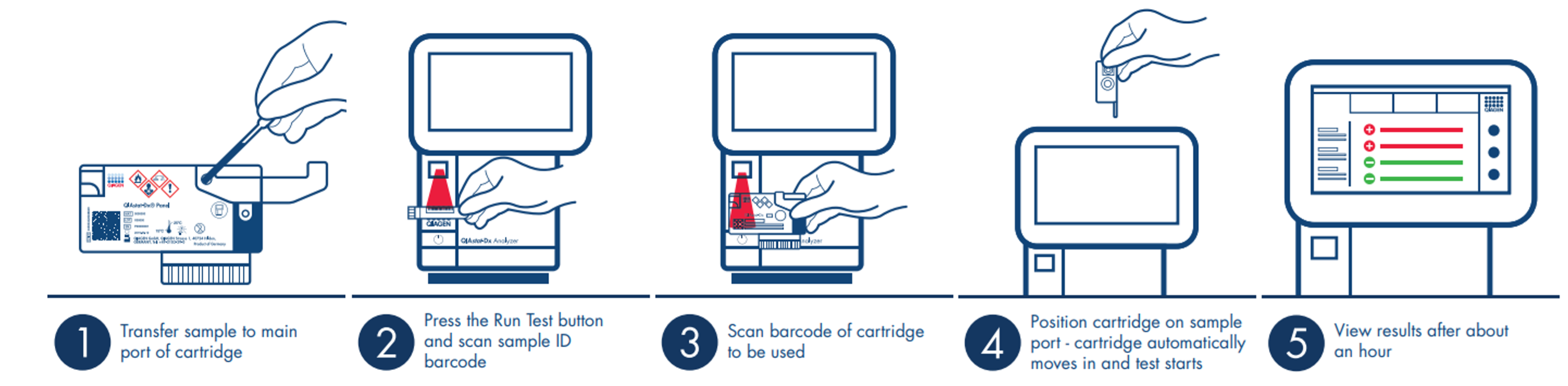
The QIAstat-Dx (DiagCORE) Analyzer is a modular and scalable system designed to work in any clinical setting. The system runs application cartridges on demand from predefined assay protocols, via displayed step-by-step instructions, offering an intuitive and safe user experience.

Preparing the QIAstat-Dx (DiagCORE) cartridge using dry swab or liquid transfer is efficient and fast. After less than a minute of hands-on prep time, insert it in the QIAstat-Dx (DiagCORE) analyzer for comprehensive results in about an hour.
Bacterial: • Mycoplasma pneumoniae • Legionella pneumophila • Bordetella pertussis
Viral: • Influenza A • Influenza A subtype H1N1/2009 • Influenza A subtype H1 • Influenza A subtype H3 • Influenza B • Coronavirus 229E • Coronavirus HKU1 • Coronavirus NL63 • Coronavirus OC43 • Parainfluenza virus 1 • Parainfluenza virus 2 • Parainfluenza virus 3 • Parainfluenza virus 4 • Adenovirus • Respiratory Syncytial virus A/B • Human Metapneumovirus A/B • Bocavirus • Rhinovirus/Enterovirus* • SARS-CoV-2
Bacterial: • Clostridium difficile toxin A/B • Enteroaggregative E.coli (EAEC) • Enteroinvasive E. coli (EIEC)/Shigella • Enteropathogenic E. coli (EPEC) • Enterotoxigenic E. coli (ETEC) It/st • Campylobacter spp. (C.jejuni, C.upsaliensis, C.coli) • Plesiomonas shigelloides • Salmonella spp.
Viral: • Adenovirus F40/41 • Astrovirus • Norovirus GI • Norovirus GII • Rotavirus A • Sapovirus (GI, GII, GIV, GV)
Parasitic: • Cryptosporidium spp. • Cyclospora cayetanensis • Entamoeba histolytica • Giardia lamblia
Bacterial: • Escherichia coli K1 • Haemophilus influenzae • Listeria monocytogenes • Neisseria meningitidis (encapsulated) • Streptococcus agalactiae • Streptococcus pneumoniae • Mycoplasma pneumoniae • Streptococcus pyogenes
Viral: • Enterovirus • Herpes simplex virus 1 • Herpes simplex virus 2 • Human herpes virus 6 • Human parechovirus • Varicella zoster virus
Fungal: • Cryptococcus • neoformans/gattii.
Salah Alden Road | Al Zahra District
Riyadh | Kingdom of Saudi Arabia
60001 Riyadh 11545